In this blogpost by Jonas Fischer, springtails were exposed to copper oxide nanoparticles at realistic field concentrations in four soils of varying properties. Toxic effects occurred only in loamy soils and mostly at the lowest test concentrations. Reduced nanoparticle agglomeration and clay-nanoparticle interactions were considered being responsible for this observation.
Copper-based nanoparticles in agriculture and soil ecotoxicology
For decades, Copper has been applied in agriculture as a fungicide and fertilizer. But it can accumulate in top soils and adversely impact exposed soil biota. Copper-based nanoparticles have now become active ingredients for agricultural formulations (e.g. Kocide3000, Funguran). Although such formulations lead to more efficient applications and reduce the amount of applied copper, they also raise novel environmental risks.
Copper oxide nanoparticles (CuO-NP) are a widely tested model substance. They exhibit high toxicity towards cells and medium toxicity towards aquatic organisms. However, negative effects towards soil invertebrates have rarely been found. Most studies have been conducted in sandy reference soils spiked with copper concentrations. These concentrations are far above what would be representative for agricultural applications. This is problematic because of two reasons:
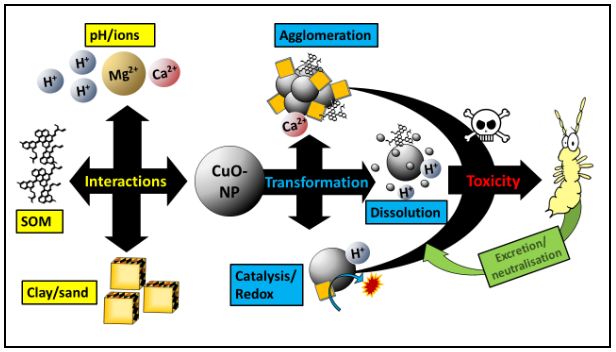
Firstly, the behaviour and the resulting risk of CuO-NP is strongly depending on their interaction with the environment. Different soil compartments, such as the organic fraction, clays or the soil solution, can cause agglomeration or dissolution of CuO-NP (Fig 1). They can also induce catalytic reactions (Fig. 1). The constant use of the same standard reference soil may fail to observe interactions causing toxicity. Therefore, a broad range of soils should be tested to represent a wide set of possible interaction partners. A special focus should be put on loamy soils. Loamy soils are widely used in agriculture due to their preferable properties to plant growth.
Secondly, applications of unrealistic concentrations skews the field behaviour of nanoparticles. The mentioned transformation processes of CuO-NP are highly concentration dependent, e.g. at lower concentrations, agglomeration is decreased while dissolution is increased. Indeed, decades of copper application have resulted in very high soil concentrations in the range of g/kg. However, due to time-dependent absorption and transformation processes in soils, freshly applied copper contributes most towards toxicity to soil organisms. Therefore, the simulation of a fresh field application with test concentrations in the low mg/kg range seems more reasonable.
The impact of soil properties on CuO-NP ecotoxicity
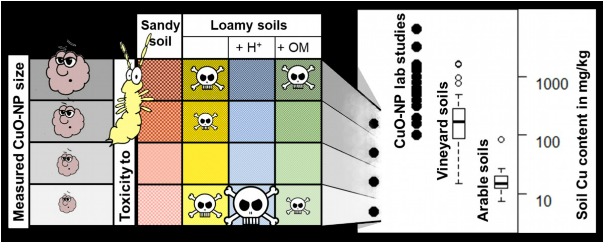
The study revealed toxic effects of CuO-NP only in loamy soils, being most pronounced at the lowest test concentration of 5 mg Cu/kg soil. Within the loamy soils, effects were highest in a loamy-acidic soil, reducing reproduction by 61% and growth by 28%. The lowest impact on reproduction was found in loamy-organic soil.
The copper concentration in soil pore water and springtail tissue was highest in the sandy soil due to its low absorption capacity. This underlines that copper availability and bioaccumulation are uncoupled from toxic effects, suggesting a specific interaction between CuO-NP and clays being toxic towards springtails.
In the group of Environmental Chemistry at the University of Landau, the researchers were able to measure the size distribution of CuO-NP in the soil pore water using single particle ICP-MS. The data clearly showed that CuO-NP size increased with test concentration. This might explain why toxicity surprisingly was most pronounced at low concentrations (Fig. 2): firstly, smaller and less aggregated NP provide more reactive surfaces per mass being able to interact with organic structures. Secondly, smaller particles are more likely retained by biological structures such fibrils which are present in the midgut epithelial cells of springtails.
However, the particle size clearly deviated between test concentrations, but not between test soils. Therefore, specific soil properties must be given to cause this low-concentration effect. In this study, a higher clay fraction seems to be most likely as all loamy soils have this in common and clays are known to form reactive associations with copper. Within loamy soils, the higher organic fraction of the loamy-organic soil may have reduced toxicity by absorption of toxic ions or coverage of reactive surfaces by soil organic matter.
Outlook
A recent unpublished study suggested that toxicity results from ROS formation induced by associations of CuO-NP and high-activity clays such as montmorillonite. Both components in combination were toxic towards springtails and caused depleted catalase activity, a sign for oxidative stress. These NP-clay associations may act as electron providers and shuttles for the formation of ROS species on the NP surface.
This hypothesis would also explain why in the previous study toxicity was highest in a loamy-acidic soil, as the described electron provision is driven by low pH. Also, the iron content in clay minerals as electron source and shuttle might play a key role. At this point, further systematic studies should be conducted to understand the complete mechanism of NP-clay interactions.
The paper “Soil properties can evoke toxicity of copper oxide nanoparticles towards springtails at low concentrations” was authored by Jonas Fischer, Anna Evlanova and Juliane Filser from the University of Bremen and Allan Philippe . It was published in Environmental Pollution.