Modern agro-ecosystems are extensively contaminated with pesticides, but the effects of these chemicals on insect biodiversity are poorly understood. Jan Erik Sedelmeir reports in this blog post about a new study, carried out at the Department of Applied Entomology at the University of Hohenheim under the direction of Georg Petschenka.
The range of studies, from lab to field, has now shed light for the first time on the effects of insecticides on grassland plant bugs, a widespread group of insects that are often found in field edges and meadows. In collaboration with Carsten Brühl, the researchers investigated how a neonicotinoid, which is still approved for outdoor use in the EU, affects plant bug populations when they come into direct or indirect contact with environmentally relevant quantities of the insecticide. The results highlight potential risks of agrochemicals to non-target insects and raise the question of whether the current risk assessment procedure is able to adequately assess environmental risks.
Not only agro-ecosystems but also nature reserves and even remote areas such as mountain ranges are widely contaminated with agrochemicals. At the same time, insect biomass, abundance, and diversity are in steep decline, with pesticide use increasingly recognized as one of the main drivers of this trend. However, despite accumulating evidence, we still know surprisingly little about the true impact of landscape-level pesticide contamination and its potential threats to even common and widely distributed insects.
Modern agricultural landscapes are complex mosaics that include not only arable fields but also often grass-dominated field margins and meadows of varying management intensity. These habitats can support diverse insect communities, among which plant bugs (Heteroptera: Miridae) are particularly abundant. This diverse and widespread family includes species that have specialized in feeding on grasses (Poaceae), making them a common component of agricultural landscapes.

Top row images: sweep net sample of a meadow next to a cereal field. The first two metres of the meadow adjacent to the field were sampled. Orange arrows indicate nymphal stages of plant bugs (Leptopterna cf. dolabrata). Bottom row images: Sweep net sample from a grass-dominated field margin of a cereal field. Orange arrows indicate nymphal stages of plant bugs (Megaloceroea cf. recticornis). All insects were subsequently released.
As Carolina Honert has just shown in her recently published paper , there is widespread and year-round contamination of agricultural landscapes with agrochemicals. It is therefore reasonable to assume that plant bugs are frequently exposed to such chemicals. Some insecticides are not only a threat through direct contact—such as contact to spray droplets or contaminated surfaces—but can also be absorbed by plants (systemic insecticides) and distributed within their tissues. This means that herbivorous non-target insects can also be exposed to these chemicals when they feed on grasses previously contaminated by pesticide drift in field margins or neighboring meadows, for example. In order to investigate these potential threats to non-target insects in such habitats, we conducted a study using pant bugs, as this widespread group of insects has been little studied in an ecotoxicological context.
We conducted field, greenhouse and laboratory experiments to mimic insecticide exposure in different scenarios. In all experiments we used a neurotoxic neonicotinoid insecticide formulation with the active ingredient acetamiprid. Acetamiprid is both a contact and systemic insecticide. Unlike other neonicotinoids, acetamiprid is still registered for open field use in the EU.
Field Experiment
In 2022, we set up a field experiment on an extensively managed meadow in the Botanical Gardens of the University of Hohenheim (Germany) to test the response of the native plant bug population to different concentrations of the neonicotinoid formulation. Twenty semi-open field plots were established in spring. The design included three treatments simulating insecticide deposition (1) inside the crop field (i.e. 100% insecticide application rate), (2) at the field margin (i.e. 30% application rate due to overspray and spray drift) and (3) at a distance of 20 m from the field (i.e. 0.15% application rate due to spray drift). In the fourth treatment, only water was applied. During application, individual enclosures were surrounded by plastic sheeting to prevent contamination of neighboring enclosures.

Bottom right image shows plastic sheeting around individual enclosures during insecticide application.
The populations of soft bugs inside the enclosures were sampled with an insect net two days after application. We were interested in both the development of the total population (nymphs and adult plant bugs) and the species-specific response, focussing on three grass-feeding species: Stenotus binotatus (Fabricius, 1794), Leptopterna dolabrata (Linnaeus, 1758) and Megaloceroea recticornis (Geoffroy, 1785).
The neonicotinoid application reduced overall plant bug abundance after two days by 83% in the field rate treatment, but also by 82% in the field margin scenario compared to the control. No significant reduction was observed for the 20 m field distance scenario. The same pattern is shown for adults of the three focal grass-feeding species.
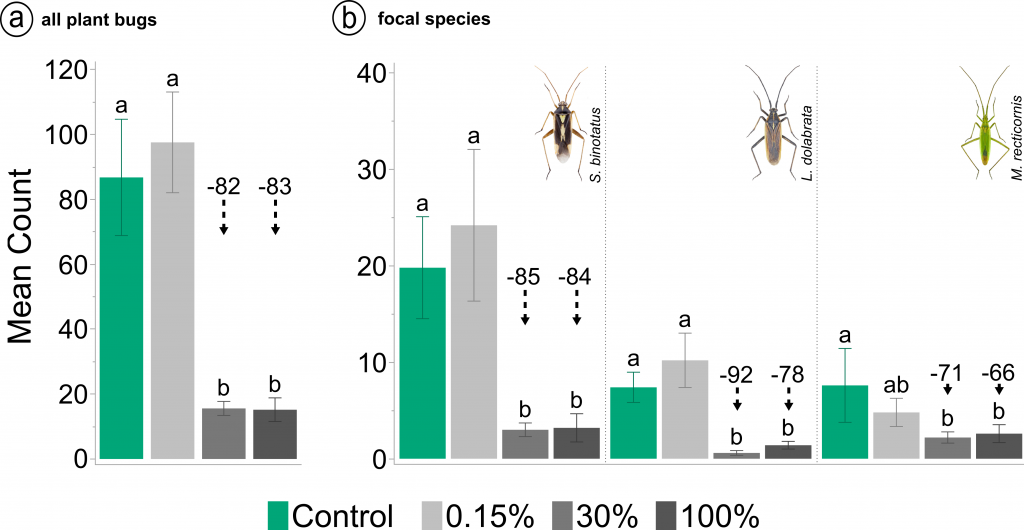
a Mean number of plant bugs (nymphs and adults) two days after insecticide application. b Mean number of adult plant bugs two days after insecticide application separated by species. Stenotus binotatus (left), Leptopterna dolabrata (centre) and Megaloceroea recticornis (right). Means not sharing the same letter are significantly different. Black dashed arrow lines in combination with numbers above bars indicate percentage difference to the control. Insect images © Gerhard Strauss, Biberach, Germany.
It was interesting to see that the differences to the control varied between species. Since this was an open field experiment, these results might have been influenced by a number of factors. First, the data could be influenced by differences in local abundance, as S. binotatus was overall more abundant than the other two species. Second, the position of a bug within the vegetation at the time of spraying could have influenced exposure. Thirdly, it could be due to a possible species-specific sensitivity to the insecticide. To further investigate the third possibility, while controlling for the others, we conducted two additional experiments using the same neonicotinoid. However, we had to wait until next year because these three species only have one generation per year and adults are only present for about four weeks.

This image was taken at a later stage, when plant bug sampling had stopped. At this stage of the experiment, additional sampling methods (i.e. photo eclectors) were applied, which are not part of this study. Photo: C. Trautmann.
In 2023, we conducted a greenhouse experiment to simulate spray drift contamination of host plants prior to plant bug migration. Additionally, we performed a laboratory experiment to simulate only direct topical contact with an insecticide droplet (at different concentrations) in controlled dose-response assays. In contrast to the field experiment, in these two experiments we were able to control for an equal distribution of individuals tested per species and treatment. We used the same neonicotinoid formulation for these experiments as we used in the field experiment.
Greenhouse Experiment
In this experiment, we simulated a scenario where plant bugs migrate to plants inside a field margin that have been previously contaminated with an insecticide. Two days after we treated host plants with the neonicotinoid according to a field edge scenario, we placed a counted number of individuals of the three focal species (S. binotatus, L. dolabrata and M. recticornis) on these plants. Four days later, we counted all living individuals again.
The results were striking: for S. binotatus, not a single individual had survived on the insecticide-treated plants, whereas almost 90% survived in the control. For the other two species, survival in the insecticide treatment was 35% and 39% lower than in the control.
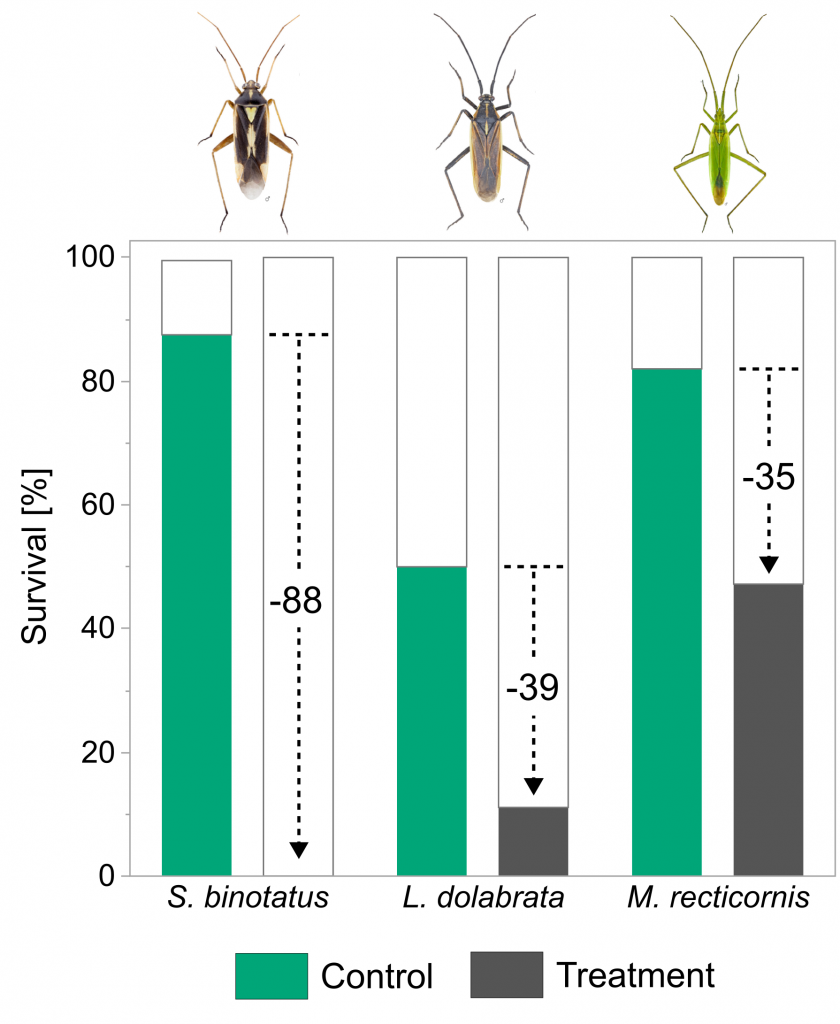
Plants were sprayed with insecticide two days prior to plant bug release. Survival rates at day four are given for Stenotus binotatus, Leptopterna dolabrata and Megaloceroea recticornis for the control (green) and the insecticide treatment (grey). Note that no individuals of S. binotatus survived the insecticide treatment and therefore no bar is shown. Black dashed lines in combination with numbers indicate percentage difference between survival in the control and treatment of the respective species. Insect images © Gerhard Strauss, Biberach.
Laboratory Experiment
To test the species-specific response to the neonicotinoid after direct contact, we conducted dose-response assays with males and females of all three species. Such assays are common tools to determine and compare species-specific sensitivity. Comparisons between species are made on the basis of the LD50 value, a concentration at which 50% of a population has died.
All three species were highly sensitive to the neonicotinoid. Furthermore, we observed the same pattern as in the greenhouse experiment, with S. binotatus being significantly more sensitive than the other two species. We then compared the LD50 values obtained in this assay with a reported value for honeybees. Based on this comparison, it is striking that the sensitivity of the tested plant bug species to this neonicotinoid is about ~11,000 times higher. Remarkably, in two of the three species, we observed more than 20-fold higher sensitivity of males compared to females.
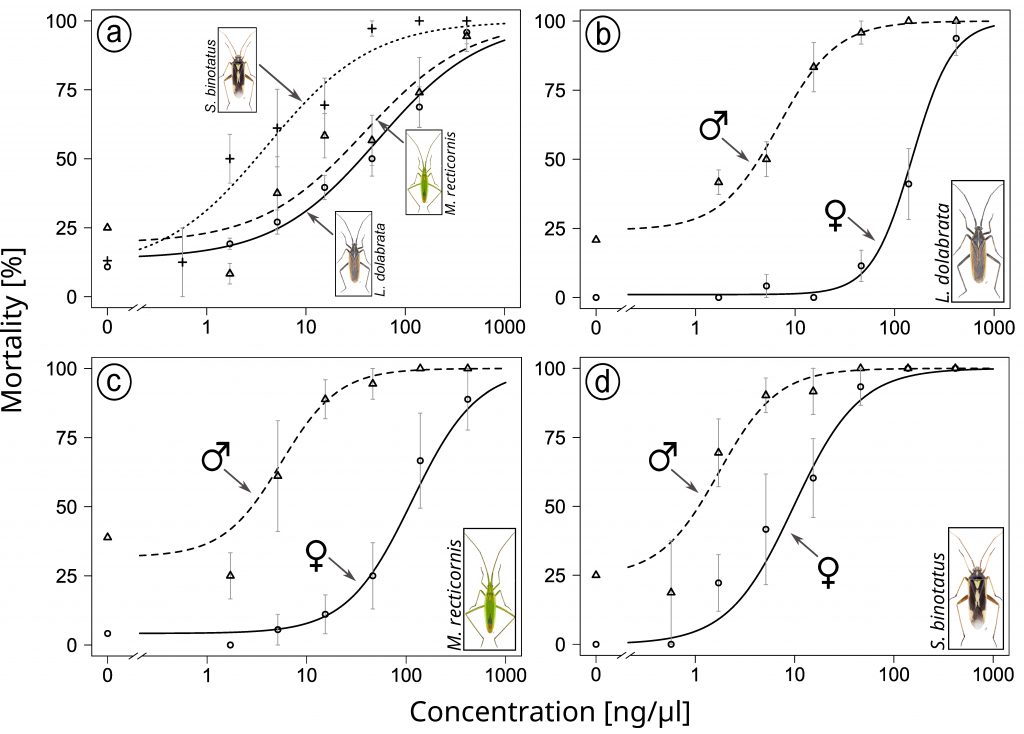
a Dose-response curves for all species, b separated by sex for Leptopterna dolabrata, c separated by sex for Megaloceroea recticornis, d separated by sex for Stenotus binotatus. Insect images © Gerhard Strauss, Biberach, Germany.
Are we drastically underestimating the environmental threat of agrochemicals?
Our study shows that plant bugs, which are present in highly exposed areas such as field margins, are physiologically very sensitive to neonicotinoids. Systemic uptake into plant tissues means that the danger posed by neonicotinoids goes beyond direct contact when the product is applied, as bugs on previously sprayed plants also have a drastically reduced survival rate. Despite this, the current risk assessment does not include plant bugs or other non-target herbivorous insects, and in Germany field margins less than 3 m wide are not considered non-target habitats for agrochemical application and are therefore not protected. Existing EU test methods do not adequately reflect the diversity of non-target insect communities, leading to a potential underestimation of environmental risks. The large differences in LD50 values – soft bugs are up to 11,000 times more sensitive than honeybees – underline this concern. In addition, current assessments ignore sex-specific sensitivity, although we found up to 22-fold sensitivity differences between males and females. In view of these results, we see an urgent need to adjust the existing risk assessment procedures for agrochemicals.
The study:
Jan Erik Sedlmeier, Ingo Grass, Prasanth Bendalam, Birgit Höglinger, Frank Walker, Daniel Gerhard, Hans-Peter Piepho, Carsten A. Brühl and Georg Petschenka 2025. Neonicotinoid insecticides can pose a severe threat to grassland plant bug communities. Communications Earth & Environment. https://www.nature.com/articles/s43247-025-02065-y.
Further study mentioned in this article:
Carolina Honert, Ken Mauser, Ursel Jäger, Carsten A. Brühl. 2025. Exposure of insects to current use pesticide residues in soil and vegetation along spatial and temporal distribution in agricultural sites. Scientific Reports. https://doi.org/10.1038/s41598-024-84811-4
